Risks Related to the Company
We have a limited operating history upon which you can evaluate our performance, and accordingly, our prospects must be considered in light of the risks that any new company encounters.
Risks Related to the Company
We have a limited operating history upon which you can evaluate our performance, and accordingly, our prospects must be considered in light of the risks that any new company encounters.
We were incorporated under the laws of the State of Nevada on August 28, 2019. We have limited operations and no operating revenue to date. We are in the development stage, and our future operations are subject to all of the risks inherent in the establishment of a new business enterprise. The likelihood of the success of our company must be considered in light of the problems, expenses, difficulties, complications and delays frequently encountered in connection with the development of an entity in the business of providing intracellular transport systems for use in delivering gene-editing chemistry, proteins, genetic materials and pharmaceutically active agents to the surface or interior of human or other cells. There can be no assurance that we will be able to generate revenues, that future revenues will be significant, that any sales will be profitable or that we will have sufficient funds available to complete our marketing and development programs or to market any new products which we may develop. We currently have operating losses, have no substantive source of operating revenue, are unable to self-finance operations, have limited resources, and there can be no assurance that we will be able to develop such revenue sources or that our operations will become profitable, even if we are able to commercialize our products and build brand awareness.
In order for the Company to compete and grow, it must attract, recruit, retain and develop the necessary personnel who have the needed experience.
Recruiting and retaining highly qualified personnel is critical to our success. These demands may require us to hire additional personnel and will require our existing management personnel to develop additional expertise. We face intense competition for personnel. The failure to attract and retain personnel or to develop such expertise could delay or halt the development and commercialization of our product candidates. If we experience difficulties in hiring and retaining personnel in key positions, we could suffer from delays in product development, loss of customers and sales and diversion of management resources, which could adversely affect operating results. Our consultants and advisors may be employed by third parties and may have commitments under consulting or advisory contracts with third parties that may limit their availability to us.
Quality management plays an essential role in determining and meeting customer requirements, preventing defects, improving the Company’s products and services and maintaining the integrity of the data that supports the safety and efficacy of our products.
Our future success depends on our ability to maintain and continuously improve our quality management program. An inability to address a quality or safety issue in an effective and timely manner may also cause negative publicity, a loss of customer confidence in us or our current or future products, which may result in the loss of sales and difficulty in successfully launching new products. In addition, a successful claim brought against us in excess of available insurance or not covered by indemnification agreements, or any claim that results in significant adverse publicity against us, could have an adverse effect on our business and our reputation.
We may implement new lines of business or offer new products and services within existing lines of business.
There are substantial risks and uncertainties associated with these efforts, particularly in instances where the markets are not fully developed. In developing and marketing new lines of business and/or new products and services, we may invest significant time and resources. Initial timetables for the introduction and development of new lines of business and/or new products or services may not be achieved and price and profitability targets may not prove feasible. We may not be successful in introducing new products and services in response to industry trends or developments in technology, or those new products may not achieve market acceptance. As a result, we could lose business, be forced to price products and services on less advantageous terms to retain or attract clients, or be subject to cost increases. As a result, our business, financial condition or results of operations may be adversely affected.
The Company’s success depends on the experience and skill of the board of directors, its executive officers and key employees.
In particular, the Company is dependent on Kurt Swogger, who is the President and CEO, and Randy Kinsel, who is the Secretary and Treasurer of the Company. The loss of Kurt Swogger and Randy Kinsel or any member of the board of directors or executive officer could harm the Company’s business, financial condition, cash flow and results of operations.
If we or our licensors are unable to obtain and maintain patent protection for our technology and products, or if the scope of the patent protection obtained is not sufficiently broad, competitors could develop and commercialize technology and products similar or identical to ours, and our ability to successfully commercialize our technology and products may be impaired.
Our success depends in large part on our (or our licensor’s) ability to obtain and maintain patent and other intellectual property protection in the United States and other countries with respect to our proprietary technology and products. Our licensors’ seek to protect our proprietary position by filing patent applications in the United States and abroad related to their novel technologies and drug candidates.
The patent prosecution process is expensive and time-consuming, and our licensors may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost, in a timely manner, or in all jurisdictions. It is also possible that our licensors will fail to identify patentable aspects of our or their research and development output before it is too late to obtain patent protection. Moreover we do not have the right to control the preparation, filing and prosecution of patent applications, or to maintain the patents, covering technology that we license from third party licensors. We may also require the cooperation of our licensors in order to enforce the licensed patent rights, and such cooperation may not be provided. Therefore, these patents and applications may not be prosecuted and enforced in a manner consistent with the best interests of our business.
The patent position of biotechnology companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation. In addition, the laws of foreign countries may not protect our rights to the same extent as the laws of the United States and our licensors may fail to seek or obtain patent protection in all major markets. For example, European patent law restricts the patentability of methods of treatment of the human body more than United States law does. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore, we cannot know with certainty whether our licensors were the first to make the inventions claimed in their owned patents or pending patent applications, or that our licensors were the first to file for patent protection of such inventions. As a result, the issuance, scope, validity, enforceability and commercial value of our or our licensors’ patent rights are highly uncertain. Our and our licensors’ pending and future patent applications may not result in patents being issued which protect our and our licensors’ technology or products, in whole or in part, or which effectively prevent others from commercializing competitive technologies and products. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our licensors’ patents or narrow the scope of our patent protection.
Recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents. On September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to United States patent law. These include provisions that affect the way patent applications are prosecuted and may also affect patent litigation. The U.S. Patent and Trademark Office, or U.S. PTO, recently developed new regulations and procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the first to file provisions, only became effective on March 16, 2013. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business and financial condition.
The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our owned and licensed patents may be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in loss of exclusivity or freedom to operate or in patent claims being narrowed, invalidated or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our technology and products. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
We may become involved in lawsuits to protect or enforce our or our licensors’ patents or other intellectual property, which could be expensive, time- consuming and ultimately unsuccessful.
Competitors may infringe issued patents or other intellectual property that is licensed to us. To counter infringement or unauthorized use, we or our licensors may be required to file infringement claims, which can be expensive and time-consuming. Any claims we or our licensors assert against perceived infringers could provoke these parties to assert counterclaims against us alleging that we or our licensors infringe their intellectual property. In addition, in a patent infringement proceeding, a court may decide that a patent of our licensor is invalid or unenforceable, in whole or in part, construe the patent’s claims narrowly or refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation proceeding could put one or more of our licensed patents at risk of being invalidated or interpreted narrowly, which could adversely affect us and our collaborators.
If we fail to comply with our obligations in our intellectual property licenses with third parties, we could lose rights that are important to our business.
We are party to a key license agreement that imposes, and we may enter into additional licensing and funding arrangements with third parties that may impose, diligence, development and commercialization timelines, milestone payment, royalty, insurance and other obligations on us. Under our existing licensing agreement, we are obligated to pay royalties of revenues to the extent they are covered by the agreement. If we fail to comply with our obligations under current or future license agreements, our counterparties may have the right to terminate these agreements, in which event we might not be able to develop, manufacture or market any product that is covered by these agreements or may face other penalties under the agreements. Such an occurrence could materially adversely affect the value of drug candidates being developed using rights licensed to us under any such agreement. Termination of these agreements or reduction or elimination of our rights under these agreements may result in our having to negotiate new or reinstated agreements with less favorable terms, or cause us to lose our rights under these agreements, including our rights to important intellectual property or technology.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patents for some of our licensed technology, we also rely on trade secrets, including unpatented know- how, technology and other proprietary information, to maintain our competitive position. We seek to protect these trade secrets, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as our employees, corporate collaborators, outside scientific collaborators, contract manufacturers, consultants, advisors and other third parties. We also seek to enter into confidentiality and invention or patent assignment agreements with our employees and consultants. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Our trade secrets may also be obtained by third parties by other means, such as breaches of our physical or computer security systems. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them, or those to whom they communicate it, from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our competitive position would be harmed.
We are subject to income taxes as well as non-income based taxes, such as payroll, sales, use, value-added, net worth, property and goods and services taxes, in the U.S.
Significant judgment is required in determining our provision for income taxes and other tax liabilities. In the ordinary course of our business, there are many transactions and calculations where the ultimate tax determination is uncertain. Although we believe that our tax estimates are reasonable: (i) there is no assurance that the final determination of tax audits or tax disputes will not be different from what is reflected in our income tax provisions, expense amounts for non-income based taxes and accruals and (ii) any material differences could have an adverse effect on our financial position and results of operations in the period or periods for which determination is made.
Successful development of our products is uncertain.
The products that we expect to develop are based on processes and methodologies that are not currently widely employed. Our development of current and future products are subject to the risks of failure and delay inherent in the development of new products and products based on new technologies, including delays in product development, testing, or manufacturing; unplanned expenditures in product development, testing, or manufacturing, a failure to receive regulatory approvals, the inability to manufacture on our own, or through any others, products on a commercial scale, or failure to achieve market acceptance, and the emergence of superior or equivalent products.
Because of these risks, our research and development efforts may not result in any commercially viable products. If a significant portion of these development efforts are not successfully completed, required regulatory approvals are not obtained, or any approved products are not commercially successfully, our business, financial condition, and results of operations may be materially harmed.
Political, economic and regulatory influences are subjecting the healthcare industry to potential fundamental changes that could substantially affect our results of operations.
Government and private sector initiatives to limit the growth of healthcare costs, including price regulation, competitive pricing, coverage and payment policies, comparative effectiveness of therapies, technology assessments and alternative payment models, are continuing in countries where we do business, including the U.S. These changes are causing the marketplace to put increased emphasis on the delivery of more cost-effective treatments. As a U.S. headquartered Company with most of our future sales being expected to come from the U.S., this healthcare reform legislation will materially impact us. Certain provisions of the legislation will not be effective for a number of years and it is unclear what the full impact of the legislation will be. Provisions of this legislation, including Medicare provisions aimed at improving quality and decreasing costs, comparative effectiveness research, an independent payment advisory board, and pilot programs to evaluate alternative payment methodologies, could meaningfully change the way healthcare is developed and delivered, and may adversely affect our business and results of operations. Further, we cannot predict what healthcare programs and regulations will be ultimately implemented at the federal or state level, or the effect of any future legislation or regulation in the U.S. or internationally. However, any changes that lower reimbursements for our products, reduce medical procedure volumes or increase cost containment pressures on us or other participants in the healthcare industry could adversely affect our business and results of operations.
Products that we manufacture, source, distribute or market are required to comply with regulatory requirements.
To lawfully operate our businesses, we are required to hold permits, licenses and other regulatory approvals from, and to comply with operating and security standards of, governmental bodies. Failure to maintain or renew necessary permits, licenses or approvals, or noncompliance or concerns over noncompliance may result in suspension of our ability to distribute, import or manufacture products or criminal and civil sanctions and could have an adverse effect on our results of operations and financial condition.
Although we are not required to register MGMR with U.S. Food and Drug Administration (“FDA”) since it is a tool used to make the active compound our future third party licenses will be subject to substantial regulation by the FDA and other regulatory authorities globally.
Since MGMR will only be used to modify cells outside the body, we are not required to register MGMR with the FDA as it is a tool to make the active compound. However, our future licensees who are the formulators of CAR-T will have to register use of MR with the FDA. Accordingly, the use of our product by third party licensees will be subject to substantial regulation by the FDA.
Any new licensee product must undergo lengthy and rigorous testing and other extensive, costly and time-consuming procedures mandated by FDA and foreign regulatory authorities. Changes to current products may be subject to vigorous review, including additional 510(k) and other regulatory submissions, and approvals are not certain. Our licensees’ facilities must be approved and licensed prior to production and remain subject to inspection from time to time thereafter. Failure to comply with the requirements of FDA or other regulatory authorities, including a failed inspection or a failure in our licensees’ adverse event reporting system, could result in adverse inspection reports, warning letters, product recalls or seizures, monetary sanctions, injunctions to halt the manufacture and distribution of licensee products, civil or criminal sanctions, refusal of a government to grant approvals or licenses, restrictions on operations or withdrawal of existing approvals and licenses. Any of these actions could cause our licensees to lose the confidence of their customers in the licensees’ products, which could adversely affect our sales and results of operations as our sales and results of operations are dependent upon royalty revenue from our clients.
The commercial success of our products will depend in part upon the level of reimbursement our licensees receive from third parties for the cost of their products to users.
The commercial success of any licensee product will depend, in part, on the extent to which reimbursement for the costs of licensee products and related treatments will be available from third-party payors such as government health administration authorities, private health insurers, managed care programs, and other organizations. Adequate third-party insurance coverage may not be available for our licensees to establish and maintain price levels that are sufficient for them to continue their business or for realization of an appropriate return on investment in product development. The result of this occurring would be to reduce our royalty revenues from our licensee customers which could have a material adverse effect on our business, financial condition and prospects.
If our future licensees are not able to obtain, or if there are delays in obtaining, required regulatory approvals, our licensees will not be able to commercialize their drug candidates or will not be able to do so as soon as anticipated, and our ability to generate royalty revenue from our licensees will be materially impaired.
Our licensees’ products and the activities associated with their development and commercialization, including their design, testing, manufacture, safety, efficacy, recordkeeping, labeling, storage, approval, advertising, promotion, sale and distribution, are subject to comprehensive regulation by the FDA and other regulatory agencies in the United States and by the EMA and similar regulatory authorities outside the United States. Failure to obtain marketing approval for our licensees’ products will prevent them from commercializing their products. We have not yet licensed our products to any licensee. Therefore, none of our future licensees have received approval to market any of their products which contain our transport mechanism from regulatory authorities in any jurisdiction. Securing marketing approval requires the submission of extensive preclinical and clinical data and supporting information to regulatory authorities for each therapeutic indication to establish the drug candidate’s safety and efficacy. Securing marketing approval also requires the submission of information about the product manufacturing process to, and inspection of manufacturing facilities by, the regulatory authorities. Our future licensees’ products may not be effective, may be only moderately effective or may prove to have undesirable or unintended side effects, toxicities or other characteristics that may preclude their obtaining marketing approval or prevent or limit commercial use. For example, new cancer drugs frequently are indicated only for patient populations that have not responded to an existing therapy or have relapsed. If our future licensee’s products with a cancer indication receives marketing approval, the accompanying label may limit the approved use of our drug in this way, which could limit sales of the product and thereby have a negative effect on the level of royalties that we receive for licensing our technology to our future licensees and also negatively impact our results of operations and financial condition.
The process of obtaining marketing approvals, both in the United States and abroad, is expensive and may take many years. If additional clinical trials are required for certain jurisdictions, these trials can vary substantially based upon a variety of factors, including the type, complexity and novelty of the products involved, and may ultimately be unsuccessful. Changes in marketing approval policies during the development period, changes in or the enactment of additional statutes or regulations, or changes in the regulatory review process for each submitted product application, may cause delays in the review and approval of an application. Regulatory authorities have substantial discretion in the approval process and may refuse to accept a marketing application as deficient or may decide that our data is insufficient for approval and require additional preclinical, clinical or other studies. In addition, varying interpretations of the data obtained from preclinical and clinical testing could delay, limit or prevent marketing approval of a drug candidate. Any marketing approval our future licensees ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the approved product not commercially viable.
The FDA and other regulatory authorities are monitoring whether nanotechnology-based therapeutics pose any specific health and human safety risks. In June 2014, the FDA issued guidance providing that it will address issues such as safety, effectiveness, public heath impact, and regulatory status of nanotechnology products on a case-by-case basis using the FDA’s existing review processes. It is possible that the FDA or other regulatory authorities could issue additional guidance or regulations in the future regarding nanotechnology-based therapeutics that could adversely affect our future licensees’ drug candidates.
If our future licensees experience delays in obtaining approval or if they fail to obtain approval of their drug candidates, the commercial prospects for their drug candidates may be harmed and their ability to generate revenues will be materially impaired, which would result in a material impairment in our ability to generate royalty revenue from them.
We face significant competition from other biotechnology companies.
Our MGMR product faces unique groupings of competitive technologies depending on the application. Not all competitive technologies are relevant in each application and market. Depending on the application, competitors technologies are associated with a unique set of advantages and disadvantages which vary in magnitude relative to MGMR. We face potential competition from many different sources, including major pharmaceutical, specialty pharmaceutical and biotechnology companies, academic institutions and governmental agencies and public and private research institutions. We also face competition from other nanomedicine platforms developing targeted therapies, including platforms focused on albumin nanoparticles, liposomes and polymeric nanoparticles.
Many of our competitors may have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical, biotechnology and diagnostic industries may result in even more resources being concentrated among a smaller number of our competitors. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies.
Our manufacturing activity is subject to certain risks.
We may manufacture the products sold to our customers in a location to be obtained in the future. As a result, we may be dependent upon the uninterrupted and efficient operation of our manufacturing facility and our distribution facilities throughout the country. Our manufacturing facilities and distribution facilities may be subject to the risk of catastrophic loss due to, among other things, earthquake, fire, flood, terrorism or other natural or man-made disasters, as well as occurrence of significant equipment failures. If any of these facilities were to experience a catastrophic loss, it would be expected to disrupt our operations and could result in personal injury or property damage, damage relationships with our customers or result in large expenses to repair or replace the facilities or systems, as well as result in other liabilities and adverse impacts.
We may contract with third-party manufacturers to produce our products in the future in accordance with our specifications and standards. These contract manufacturers are subject to the same risks as our manufacturing facility as noted above. While we plan to implement stringent quality control procedures to verify that our contract manufacturers comply with our specifications and standards, we will not have full control over their manufacturing activities. Any difficulties, delays and defects in our products resulting from the activities of our contract manufacturers may have an adverse effect on our business and results of operations.
We are dependent on our collaborative agreements for the development of products and business development, which exposes us to the risk of reliance on the viability of third parties.
In conducting our research and development activities, we will in the future rely on collaborative agreements with third parties such as manufacturers, contract research organizations, commercial partners, universities, governmental agencies and not-for-profit organizations for both strategic and financial resources. The loss of, or failure to perform by us or our partners under, any applicable agreements or arrangements, or our failure to secure additional agreements for other products in development, would substantially disrupt or delay our research and development and commercialization activities. Any such loss would likely increase our expenses and materially harm our business, financial condition and results of operation.
Reliance on third-party relationships and outsourcing arrangements could adversely affect our business.
We utilize third parties, including suppliers, alliances with other pharmaceutical and biotechnology companies, and third-party service providers, for selected aspects of product development, the manufacture and commercialization of certain products, support for information technology systems, and certain financial transactional processes. Outsourcing these functions involves the risk that the third parties may not perform to our standards or legal requirements, may not produce reliable results, may not perform in a timely manner, may not maintain the confidentiality of our proprietary information, or may fail to perform at all. Failure of these third parties to meet their contractual, regulatory, confidentiality, or other obligations to us could have a material adverse effect on our business.
The forecasts of market growth included in our business plan and investor presentations may prove to be inaccurate, and even if the markets in which we compete achieve the forecasted growth, we cannot assure you our business will grow at similar rates, if at all.
Growth forecasts are subject to significant uncertainty and are based on assumptions and estimates that may not prove to be accurate. The forecasts in our business plan and investor presentations may prove to be inaccurate. Even if these markets experience the forecasted growth described in our business plan, we may not grow our business at similar rates, or at all. Our growth is subject to many factors, including our success in implementing our business strategy, which is subject to many risks and uncertainties. Accordingly, the forecasts of market growth included in our business plan should not be taken as indicative of our future growth.
We will need additional financing to execute our business plan, which we may not be able to secure on acceptable terms, or at all.
We will require additional financing in the near and long term to fully execute our business plan. Our success depends on our ability to raise such additional financing on reasonable terms and on a timely basis. Conditions in the economy and the financial markets may make it more difficult for us to obtain necessary additional capital or financing on acceptable terms, or at all. If we cannot secure sufficient additional financing, we may be forced to forego strategic opportunities or delay, scale back or eliminate further development of our goals and objectives, operations and investments or employ internal cost savings measures.
We plan to obtain insurance that may not provide adequate levels of coverage against claims.
We plan to obtain insurance customary for businesses of our size and type. However, there are types of losses we may incur that cannot be insured against or that we believe are not economically reasonable to insure. Such losses could have a material adverse effect on our business and results of operations.
Risks Related to the Company’s Securities and this Offering
Affiliates of our company, including officers, directors and existing stockholder of our company, may invest in this offering and their funds will be counted toward our achieving the minimum amount.
There is no restriction on our affiliates, including our officers, directors and existing stockholders, investing in the offering. As a result, it is possible that if we have raised some funds, but not reached the minimum amount, affiliates can contribute the balance so that there will be a closing. The minimum amount is typically intended to be a protection for investors and gives investors confidence that other investors, along with them, are sufficiently interested in the offering and our company and its prospects to make an investment of at least the minimum amount. By permitting affiliates to invest in the offering and make up any shortfall between what non-affiliate investors have invested and the minimum amount, this protection is largely eliminated. Investors should be aware that no funds other than their own and those of affiliates investing along with them, may be invested in this offering.
We intend to use some of the proceeds from the offering for unspecified working capital.
This means that we have ultimate discretion to use this portion of the proceeds as we see fit and have chosen not to set forth any specific uses for you to evaluate. The net proceeds from this offering will be used for the purposes, which our management deems to be in our best interests in order to address changed circumstances or opportunities. As a result of the foregoing, our success will be substantially dependent upon our discretion and judgment with respect to application and allocation of the net proceeds of this offering. We may choose to use the proceeds in a manner that you do not agree with and you will have no recourse. A use of proceeds that does not further our business and goals could harm our company and its operations and ultimately cause you to lose all or a portion of your investment.
We are not subject to Sarbanes-Oxley regulations and lack the financial controls and safeguards required of public companies.
We do not have the internal infrastructure necessary, and are not required, to complete an attestation about our financial controls that would be required under Section 404 of the Sarbanes-Oxley Act of 2002. There can be no assurance that there are no significant deficiencies or material weaknesses in the quality of our financial controls. We expect to incur additional expenses and diversion of management’s time if and when it becomes necessary to perform the system and process evaluation, testing and remediation required in order to comply with the management certification and auditor attestation requirements.
The securities being sold in this offering will not be freely tradable until one year from the initial purchase date. Although our securities may be tradable under federal securities law, state securities regulations may apply, and each investor should consult with his or her attorney.
You should be aware of the long-term nature of this investment. There is not now and likely will not be a public market for our securities. Because our securities have not been registered under the Securities Act or under the securities laws of any state or non-United States jurisdiction, our securities have transfer restrictions and cannot be resold in the United States except pursuant to Rule 501 of Regulation CF. It is not currently contemplated that registration under the Securities Act or other securities laws will be effected. Limitations on the transfer of the securities may also adversely affect the price that you might be able to obtain for our securities in a private sale. Investors should be aware of the long-term nature of their investment in the Company. Each investor in this offering will be required to represent that it is purchasing the securities for its own account, for investment purposes and not with a view to resale or distribution thereof.
Neither the offering nor the securities have been registered under federal or state securities laws, leading to an absence of certain regulation applicable to us.
No governmental agency has reviewed or passed upon this offering, our company or any Securities of our company. We also have relied on exemptions from securities registration requirements under applicable state securities laws. Investors, therefore, will not receive any of the benefits that such registration would otherwise provide. Prospective investors must therefore assess the adequacy of disclosure and the fairness of the terms of this offering on their own or in conjunction with their personal advisors.
No Guarantee of Return on Investment
There is no assurance that an investor will realize a return on its investment or that it will not lose its entire investment. For this reason, each investor should read the Form C and all Exhibits carefully and should consult with its own attorney and business advisor prior to making any investment decision.
A majority of our company is owned by a small number of owners.
Prior to the offering our officers, directors and those of our stockholders who own ten percent or more of our securities collectively own directly or indirectly 100% of our company. Subject to any fiduciary duties owed to our other owners or investors under Delaware law in the case of our officers and directors, these stockholders may be able to exercise significant influence over matters requiring owner approval, including the election of directors or managers and approval of significant company transactions, and will have significant control over our management and policies. These control persons may have interests that are different from yours. For example, they may support proposals and actions with which you may disagree. The concentration of ownership could delay or prevent a change in control of our company or otherwise discourage a potential acquirer from attempting to obtain control of the Company, which in turn could reduce the price potential investors are willing to pay for our company. In addition, this owner could use his voting influence to maintain the Company’s existing management, delay or prevent changes in control of our company, or support or reject other management and board proposals that are subject to owner approval.
We have the right to extend the offering deadline.
We may extend the offering deadline beyond what is currently stated herein. This means that your investment may continue to be held in escrow while we attempt to raise the minimum amount even after the offering deadline stated in this offering statement is reached. Your investment will not be accruing interest during this time and will simply be held until such time as the new offering deadline is reached without our company receiving the minimum amount, at which time committed funds will become immediately available for withdrawal from the investor’s brokerage account maintained with the Intermediary without interest or deduction, or until we receive the minimum amount, at which time it will be released to us to be used as set forth herein. Upon or shortly after release of such funds to us, the securities will be issued and distributed to you.
Your ownership of the shares will be subject to dilution.
If we conduct subsequent offerings of securities, issue shares pursuant to a compensation or distribution reinvestment plan or otherwise issues additional shares, investors who purchase securities in this offering who do not participate in those other stock issuances will experience dilution in their percentage ownership of our company’s outstanding shares. Furthermore, shareholders may experience a dilution in the value of their underlying shares depending on the terms and pricing of any future share issuances (including the underlying shares being sold in this offering) and the value of the our assets at the time of issuance.
Management has discretion over proceeds of this offering.
We expect to use the net proceeds of this offering, over time, for general marketing and advertising, leasing costs, debt repayment and general working capital. However, we have no current specific plans for the net proceeds of this offering other than as outlined in the use of proceeds section of this offering statement. As a result, our management will have the discretion to allocate the net proceeds to uses that investors may not deem desirable. There can be no assurance that the net proceeds can or will be invested to yield a significant return.
The securities will be equity interests in our company and will not constitute indebtedness.
The securities will rank junior to all existing and future indebtedness and other non-equity claims on our company with respect to assets available to satisfy claims on the Company, including in a liquidation of our company. Additionally, unlike indebtedness, for which principal and interest would customarily be payable on specified due dates, there will be no specified payments of dividends with respect to the securities and dividends are payable only if, when and as authorized and declared by us and depend on, among other matters, our historical and projected results of operations, liquidity, cash flows, capital levels, financial condition, debt service requirements and other cash needs, financing covenants, applicable state law, federal and state regulatory prohibitions and other restrictions and any other factors our board of directors deems relevant at the time. In addition, there is no limit on the amount of debt or other obligations we may incur in the future. Accordingly, we may incur substantial amounts of additional debt and other obligations that will rank senior to the securities, which are the most junior securities of our company.
There can be no assurance that we will ever provide liquidity to investors through either a sale of our company or a registration of the securities.
There can be no assurance that any form of merger, combination, or sale of our company will take place, or that any merger, combination, or sale would provide liquidity for investors. Furthermore, we may be unable to register the securities for resale by investors for legal, commercial, regulatory, market-related or other reasons. In the event that we are unable to effect a registration, investors could be unable to sell their securities unless an exemption from registration is available.
The offering price in this offering may not represent the value of our securities.
The price of the securities being sold in this offering has been determined based on a number of factors and does not necessarily bear any relationship to our book value, assets, operating results or any other established criteria of value. Prices for our securities may not be indicative of the fair market value of our securities now or in the future.












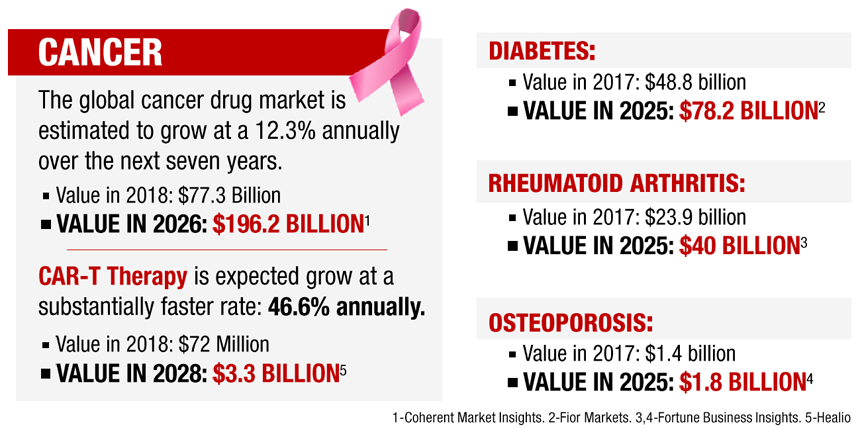










Are you licensing this technology from someone and if so how long do you have the rights?
Hiw much have you raised so far this CF round?
Michael, thanks for the question. BioPact CT licensed the technology from Molecular Rebar Design,LLC which owns a significant equity interest in this new company. There are several patents that cover this effort; a key patent expires in 2037 or about 17 years. This is the first day of the offer so you could be the first investor! We appreciate your interest. Regards, Kurt
Hi. I’m very interested in investing but I have a few questions:
-How do you make money? Selling software, subscriptions, special equipment, by procedure, etc?
-Can you give me your revenue projections for the next 5 years? If you are not allowed to disclose it then can you please share your revenue goals for that period of time?
-What’s your fundraising goal and how much has been raised so far?
-When does this offering close?
Thanks.
Leonardo, thanks for the question. BpCT will make money on licenses which have milestone payments, royalties and product sales. Each license is likely to be a bit different and later licenses will have higher payments on milestones. Our 5 year sales forecast is $19 million with an 80% margin. The offering closes June 1, 2020.
Best regards, Kurt
Is there a investment window when does it close?
Hi Sean, thank you for the question.
The last date of the offering at this time is June 30, 2020.
what is the minimum investment?
Hi Sheldon, thanks for the question. The minimum investment is $455 which is 300 shares.
Kurt, BioPact CT is fully owned by BioPact Ventures, LLC, correct? I don’t see anything in the financials about Molecular Rebar Design, LLC. Please explain the relationship.
Hi William, thank you for the question. BioPact CT was fully owned by BioPact Ventures prior to this offering. Molecular Rebar Design, LLC owns about 50% of BioPact Ventures, LLC and licensed the rights to use MGMR in the medical field to BioPact Ventures which in turn licensed the rights to BioPact CT for the use in outside the body cellular transport.
Do you need FDA approval and if so when do you plan to apply? Do you have any projections?
Dear Guenadi,
Thank you for the question. MGMR is a microscopic nanosize tool used during the process of making products such as CAR-T. MGMR does not enter the body, so consequently, it does not require FDA approval. The final product such as CAR-T does need FDA approval, but the drug company has to obtain that registration. MGMR only has to meet the specifications set by BioPact CT and the drug maker. BioPact CT will target companies that have or will have product registrations to more quickly ramp up sales and milestone payments.
Hello Kurt,
Clarification needed:
In the Q&A section, there are two dates listed as being the “Last day of Offering”:
First date: February 27th states June 1, 2020
Second date: March 3, 2020 states June 30, 2020.
Please advise as to which date is correct.
Also, if one has already invested, am I correct in that one has up to the last day of offering to do so?
Thank you in advance.
Ginny
Dear Virginia,
Excellent catch! The correct date is June 30, 2020 and you can invest up to and on that date. We made an error in my response to Leonardo and I apologize for that.
Rest regards, Kurt
Kurt, If one wishes to add to their present commitment, would they use the same Investor ID to add to that particular account? Or would one just sign up again? Best Regards, Jerome
Hello Jerry,
Thank you for your question. To make an additional investment, you should login into your account using your email and password. Click on the “invest now” button and complete the forms again.
This insures that each subscription agreement and transaction id is crystalized upon completion.
Hope this help.
Regards,
Kurt
Can your product have any possible benefit toward helping ease the effects of the COVID 19 pandemic? What other maladies might be helped by your product?
Hi John,
Thanks for the question. We are looking at this problem and have identified some approaches. This work and efforts would not be part of BioPact CT, as this new effort is not part of the scope within cellular transport arena. Our main focus will remain with progressing cellular transport.
Regards, Kurt
Where was your article published, in August 2019? Can you provide a link so that I could read this article?
Hi Anthony,
Thank you for your interest and question.
You can find a link to the original article on our website here… https://biopactct.com/results/#publications , if you or any other potential investors would like to review.
Thank you for your interest and for considering investing in BioPactCT.
Best Regards,
Kurt Swogger
Hi… When will be the IPO date?
Thank you for your question. Our plans for the future are to grow as much as reasonably possible so that we have options in 2-4 years to take the company to the next level. When we get there, we will carefully evaluate all opportunities and do what is in the investors’ best interest. This may be a public offering, or it may be an acquisition by a larger company, or we can continue to grow the company and pay dividends to shareholders as we grow.
I hope this helps to clarify the situation.
Thank you,
Kurt
What exchange and when will the IPO go live in the stock market?
Hi David,
Our plans for the future are to grow as much as reasonably possible so that we have options in 2-4 years to take the company to the next level. When we get there, we will carefully evaluate all opportunities and do what is in the investors’ best interest. This may be a public offering, or it may be an acquisition by a larger company, or we can continue to grow the company and pay dividends to shareholders as we grow.
I hope this helps to clarify the situation.
Thank you,
Kurt
I have never invested before. I couldn’t find IF the amount of shares($$) is only ONE time to buy your shares then just wait and watch?
or
is the minimum $$ EVERY MONTH?
Thanks
Teresa
Hello Teresa,
Thank you for the question. You would invest only the one time for an amount of shares. And then you can wait and watch. Of course if you wanted to invest again, that is another transaction but there is no requirement to do so.
Best Regards, Kurt
TY Kurt for the answer to my question.
I’ve gone over the investor information and I’m wanting to be able to get the most $$ when possible.
I’m just so confused to which way to invest would be best.
Hello,
I understand your goals and what you hope to achieve. I’m curious though, with so much money available in the sectors pertaining to medicine, why is it necessary for you to come here for investors? Something that sounds so promising would seem to have significant financial backing from the already established medical community.
Dear Robert,
Thank you for your interest and excellent question. We decided to take this investment route to allow more people to have an opportunity for this early stage venture and to avoid the venture capital environment. A traditional venture capital raise includes continual monitoring and often times, a loss of control. We chose this option as we thought it to be more efficient with the relatively small amount of capital required. A venture capital raise requires a larger minimum in capital investment and typically a larger raise in total, which we do not need at this time. Hence, crowdfunding as we have done.
Regards, Kurt
This is my first opportunity to invest in a pre -ipo. I remember when crispr went public. I got stuck sitting on the sidelines. I want to pull the trigger this time. Why do you think it will take that long to catch fire?
Hi James,
Thank you for your interest in our offering and question. Getting new drugs or treatments to the market is always a long process. Our MGMR actually does not go into the body so we do not have to register with the FDA directly. We are an intermediate that allows for the delivery of genetic engineering “tools” into the cell; so companies developing new drug therapies would use MGMR to drastically improve their process. MGMR offers many benefits including efficiency in time and cost, but time will be required to work through this new manufacturing procedure with companies that need to comply with the FDA. BopactCT’s vision is to replace the common process and methods of making the new cellular medicines such as the CAR-T treatments.
Regards, Kurt
How many Lifes do you expect to save, say in five years, at a cheaper rate?
Hi Lisa,
Thank you for your interest and question.
While we cannot predict the future, our goal is to reduce treatment cost and time by up to 50%. This reduction should allow for much wider usage in the cases where all other methods have failed. The current cost is over $400,000 per treatment. Last year, as best can be measured, only about 750 people received this therapy. Our internal models estimate 5-10 times more uses if we can drive the costs down. In 10 years with the current costs it is estimated the number of people treated to be 10 times or 7500 people. Our goal is to do 5-10 times that number.
Predicting the future is not easy, but as an engineer, I know that lower costs will allow more access by more people.
Regards, Kurt
Do you have an exit strategy for investors if the process does not go as planned?
Hi Larry,
Thank you for your interest and question. Our current exit strategy is for a corporate buyout in a few years, assuming everything goes as planned. Unfortunately, no one can foresee the future, but we have seen incredible preliminary results.
A risk is part of any investment.
Regards, Kurt
I presently have a brokerage account with Charles Schwab. I am constantly looking at values of my portfolio. Is it easy to buy or sell. How do I know the value of my 300 shares? Does it change from day to day? Once in, can I buy additional shares at any time?
Dear Henry,
Thank you for the interest and the questions. Since this is a private placement, the value of the shares will not fluctuate nor is there an active market where you can buy and sell shares. The offering terms are established and can be reviewed in our Form C. Buying shares is easy, simply register with Equifund CFP, click invest now and follow the prompts. You can buy additional shares at any time during our regulation crowdfunding offering.
Best, Kurt
I’m in but have some questions Will I receive some certificate or receipt indicating the shares owned? Reg CF was limited to 1 million dollars of fundraising by the SEC and may be raised to 5 million, what is your fundraising goal? Lastly, as early investors will we possibly be offered opportunities with you ahead of the general public?
Thanks in advance I am looking for ballpark answers none of will be considered legally binding…disclaimer
Hi Edmond,
Thank you for your message and we’re extremely happy to have you join our ever growing shareholder base. When the offering closes, you will received an executed share purchase agreement for your records. Equifund CFP will send you a ceremonial stock certificate and you will be added to our cap table via book entry (common practice for private companies). We can raise up to $1,070,000 via regulation crowdfunding. Should we look to do another raise, we always start by reaching out to our current shareholder base.
Best, Kurt.
Aloha Biopact CT, I AM wanting to invest but was concerned about your need for my social security number.
Hi Gary,
Equifund CFP requires all investors to disclose their detailed information as a matter of compliance and meeting AML/KYC mandates.
We want to make sure investors get the shares they paid for.
Thank you for the consideration.
Kurt
I would like to invest but, the application page will not turn to the other page. It keeps repeating: “must by divisible by $1.55.
Hi Jeanot,
The Biopact Cellular Transport offering is $1.55 per share, therefore the total invested amount must be dividable by the share price.
Thank you for your interest,
Kurt
Can I purchase more then 300 shares? What is the maximum I could invest?
Thank you for your interest Carl. The minimum invest is 300 shares but you are free to explore a more significant position in Biopact Cellular Transport. Signing into the investment portal will guide you to the maximum amounts you are allowed to invest (they do vary depending on how you fill out the application per SEC guidelines)
Regards,
Kurt
Do you need to be a US resident/citizen in order to be able to invest?
Thank you for your interest Said.
At this time the Biopact CT offering is only US domestic residents, over the age of 18.
Realistically, how many years do you expect this product to show a profit and make this a very wise investment? I’m 67 and would like to show a profit before I turn 72. If I were still working I would place this product in my IRA. J.Michael Cranford
Hi John,
Thank you for the interest, great question!
I can relate as I am also 70 and want to see this succeed as a wise investment. As you know there is no predicting the future, and I always advise consulting with a financial planner, as we can not and do not offer financial advice, but typical favorable results are in the 3-5 year range. Remembering, there is risk involved in any of these investments. The math is conclusive as to its utility and as we mentioned, are working with several partners in genetic engineering.
Regards, Kurt
Can someone living outside the USA can invest in the company?
Hi Kurt,
I am really interested to invest .I live outside the USA.
Could you kindly advise if I may invest to your company?
Hello Franck,
Thank you for your interest and questions.
At this current time, we’re only accepting domestic investments. If that changes, we will certainly
send out a notification to Equifund members.
Regards,
Kurt
When does the offer closes?
Hi Jerry,
Biopact CT has filed with the SEC to extend the current offering until February 12, 2021.
Thank you for your interest,
Kurt
I just join today, am late?
Hi Idalina,
Thank you for your interest!
Biopact CT has filed with the SEC to extend the current offering until February 12, 2021.
Regards,
Kurt
1) Do you have CPA financial statements.
2) What is the investment of management/employees. Do they have stock options?
3) Does management have the financial capacity to invest additional capital?
4) Does the company have bank loans and a capacity for additional borrowings?
5) What are the trade terms?
I have yet to read the company’s information.
Hi Tim,
This summary is a follow-up to the conversation you had previously.
To answer your questions in order 1) We do have CPA financial statements although since the company started late last year there is little activity in 2019 (a deposit of $3000 and an expense for checks). 2) The Management are owners of the shares and have put little into BioPact BCT but over $5 million into BioPact Ventures, the company that has spun out BioPact CT. There are no stock options. 3) Management has been contributing capital for years and will continue to do so as needed. 4) The Company has a loan from the parents of $ 200,000 payable when the Company can pay without causing a financial burden on the Company. 5) The terms on the loan are Fed Rates with a duration determined by the Board.
Thank you for your interest and time.
Regards, Kurt
When do you anticipate/estimate going public?
Hi David,
Thank you for your interest and question.
Our plans for the future are to grow as much as reasonably possible so that we have options in 2-4 years to take the company to the next level. At that time, we will carefully evaluate all opportunities and do what is in the investors’ best interest. This may be a public offering, or it may be an acquisition by a larger company, or we can continue to grow the company and pay dividends to shareholders as we grow.
Thank you,
Kurt
The date of last offering, was June 30, I just got this email, today is July 27th is it to late to, invest, if so when does the Company go public.
Hi Darren,
The offering was originally to close June 2020, but we have extended the dates until February of 2021.
Thank you for your interest,
Kurt
Are you growing the company and paying dividends to the shareholders now?
Hi Robert,
Thank you for the question and interest. BioPact CT is just starting and is not earning revenue yet so it is not paying a dividend. As to growing, our definition for growing is adding licensee and customer testing programs. We will be announcing progress on both fronts within a short while as there is a great need for our material’s ability to allow genetic and cellular engineering.
Best regards,
Kurt Swogger, CEO
Why was the offering date extended? How close to goal were you at on June 30th? Thanks
Hi Rae,
Thank you for your interest and question. The offering was extended until Feb 2021 based on investor interest and the impact Covid had between March – May. We were able to achieve their minimal goal continues to raise capital at this time.
Best Regards,
Kurt
Kurt,
After reading each of the questions and responses I have none to add…but instead have a kudos.
I am impressed at the quality, clear and concise points and expediency of your answers…if this is any indication of how hard you’ll work for those in need of this breakthrough and for your investors that believe in you…I think we will all do well.
Thanks.
Billy C.
Hi Billy,
Thank you for the kind note. We aim for clarity with no buzz words and simple explanations. We find complicated, obtuse responses mean the person(s) answering likely cannot keep it simple. Simple is much more likely to be executed and succeed. Thanks for the time and interest. I believe you will find this to be a sound investment in helping with treatment of many diseases.
Kind Regards,
Kurt
Hello, Mr. Swogger,
Your very latest bit of news concerning your collaboration with a major Biotech company is especially exciting..!! The news was delivered to me through an e-mail by Equifund. I should think this will be a big step financially for Biopact Cellular Transport, and of great benefit to all concerned. I look forward to more details as they develop in this partnership, as this could be that big break-through that all start-ups look for. Congratulations on this positive news and thank you very much for this update…!!! Continued good fortune to you..!!
Best regards,
Ron Dilks, investor
Dear Ronald,
Thank you for taking the time to send a note and offering your congratulations. We are very pleased to see the progress and are sure there is more to come. Thank you for your investment. I believe we will make a big difference in making genetic engineering very real and very effective.
Best regards,
Kurt
I would like to know what you mean by a major partnership signed up with Biopact. I am an original investor when signed up with equifund platform for your company in the infancy stage.Does this new announcement mean that you have a venture capitalist signed on or that you are being acquired shortly by this company or another company? It would give me info as to further investment as appeared in my email but need to know if status has changed in any way with new partnership. Thankyou
Hi Robert,
Thank you for the question. The announcement is related to a customer who will trial our material and if successful, as we suspect, will take a license. I think we need a few more successful trials prior to adding venture capital or an acquisition to drive up our value. This customer is the first and will not be the last.
The status of your investment is even more positive than when you invested.
Best regards,
Kurt
Can the share certificate be passed on to my heirs?
Mr. Rech,
Below are the transfer and resale rules regarding shares purchased under Regulation Crowdfunding (from the SEC’s website: https://www.sec.gov/info/smallbus/secg/rccomplianceguide-051316.htm)
– Securities purchased in a crowdfunding transaction generally cannot be resold for a period of one year, unless the securities are transferred:
(1) to the issuer of the securities;
(2) to an “accredited investor”;
(3) as part of an offering registered with the Commission; or
(4) to a member of the family of the purchaser or the equivalent, to a trust controlled by the purchaser, to a trust created for the benefit of a member of the family of the purchaser or the equivalent, or in connection with the death or divorce of the purchaser or other similar circumstance.
Dear Kurt
I’m sure you have generated some data and published it somewhere. I will appreciate it if you could direct me to a publication where you have done some comparative analysis of this technology in difficult to diffuse molecules (small and large) with/ without BpCT technology in vitro and in vivo. Does this improve the bioavailability of drug molecules/help decrease effective dose?
Regards
Sanjeev
Dear Sanjeev,
Thank you for taking the time to ask a question and the interest in our technology. Our BioPact CT site has a paper you can reference here… https://biopactct.com/results/#publications
Happy reading! Again, thank you for considering investing.
Kind regards,
Kurt
Good luck. I come from Malaysia. Can I invest ?. I am also a small investor.
Hello Mohd,
Thank you for your interest and question.
At this time, we’re only accepting domestic investments. If that changes, we will certainly
send out a notification to Equifund members.
Regards,
Kurt
Have you conducted clinical trials and if so where have they been conducted.
Dear Nicholas,
Thank you for the interest in our offering and your question.
Since our material never enters the body, BioPact CT will not do clinical trials. Our materials are administered outside the body to cell cultures which are then genetically modified with the assistance of the MGMR system. Once the cells are the right number after growth of the modified cell culture, the MGMR is removed. We have worked with and are currently working with customers to do this genetic engineering but we, as a company do not. However, this may happen with one of our customers in the future.
Regards, Kurt
International investor here.
What a shame that you do not allow non US residents to invest in your crowdfunding campaign.
Hope you will open to everyone still for this round.
Thanks,
Bruno
Accepting international investors is a little trickier than some make it out to be.
The Regulation Crowdfunding rules were written by the SEC and designed for investors and Issuers in the United States. Unfortunately, there was no section dedicated to foreign investment. Current precedent states that if an Issuer accepts foreign investment through their REG CF offering, the Issuer must have intimate knowledge of the local securities law to ensure the investor is meeting local investing guidelines. Moreover, the Issuer must ensure the CF offering also complies with securities laws of the jurisdiction in which the securities are sold. As you can see, this can create a significant compliance burden for the Issuer and the portal who’s listed the offering. We continue to work on the matter and are committed to finding a viable solution for our members.
Another international investor
A world wide spread of investors wouldn’t be bad for the company.
As well. It’s a shame that only US citizens are allowed to participate.
regards
Beni
Beni,
Regarding international investors – (forgive the repetition)
The Regulation Crowdfunding rules were written by the SEC and designed for investors and Issuers in the United States. Unfortunately, there was no section dedicated to foreign investment. Current precedent states that if an Issuer accepts foreign investment through their REG CF offering, the Issuer must have intimate knowledge of the local securities law to ensure the investor is meeting local investing guidelines. Moreover, the Issuer must ensure the CF offering also complies with securities laws of the jurisdiction in which the securities are sold. As you can see, this can create a significant compliance burden for the Issuer and the portal who’s listed the offering. We continue to work on the matter and are committed to finding a viable solution for our members.
Hi
I am a New Zealand based professional and accredited investor and have attempted to invest via this Equifund portal. When I enter my country in the form I received a warning my country must be in the US.
Are you only accepting US based investors???
Regards
Murray Hollings
Sorry I have just read the earlier comments. Disappointing and I will report this back to Andy Gordon
Regards
Murray
Mr. Hollings,
Hopefully, the statement below can clarify the challenge.
The Regulation Crowdfunding rules were written by the SEC and designed for investors and Issuers in the United States. Unfortunately, there was no section dedicated to foreign investment. Current precedent states that if an Issuer accepts foreign investment through their REG CF offering, the Issuer must have intimate knowledge of the local securities law to ensure the investor is meeting local investing guidelines. Moreover, the Issuer must ensure the CF offering also complies with securities laws of the jurisdiction in which the securities are sold. As you can see, this can create a significant compliance burden for the Issuer and the portal who’s listed the offering. We continue to work on the matter and are committed to finding a viable solution for our members.
Hi, I am somewhat familiar with what you are doing. I have been invested in Sangamo for some time. They actually seem to be ahead of the Crisper biotechs. Is Sangamo and their technology using viruses for HeMo A, Sickle Cell, Fabry disease and many others something you will work with? They use zinc fingers. Thanks
Hi Darren,
Thank you for the comment and the interest in BioPact CT. We have reached out to Sangamo as they seem to be a perfect fit for a potential customer, as they are a practitioner of CAR-T technology and are waiting for a reply.
We will continue to pursue them in hopes of showing how we can contribute to their research and programs.
Best regards,
Kurt
Can you explain the difference between your delivery method compared to “MCM”, they appear to be similiar in how they work…. Thanks
Dear Frank,
Thank you for your question and your interest in BioPact CT. Actually mRNA is what MGMR would deliver into the cell. mRNA is used to change how the cell systems work; MGMR transports it inside the cell allowing the mRNA to do its task. An advantage of MGMR is that it can deliver a variety of mRNA species (there are many) as well as other cell changing entities rather than the mRNA.
You would be investing in a tool for many systems such a mRNA allowing broad use of our product.
Regards,
Kurt
Have you any more recent literature on the use of MGMR systems for genetic modification? I would expect this, if it works as simply and effectively as you say it does, to be the “holy grail “ of delivery systems of which there are many. Not certain that I understand how this ex vivo (in vitro) technique does not result in carbon nanoparticle contamination of these cells. Is it the genetically modified nanotube -free progeny that are returned to the host?
Dear Evan,
Thank you for the question and your interest. You are correct, the genetically modified progeny, that are carbon nanotube free, are injected back into the patient. One of the final steps of the process, to make the progeny or daughter cells, the cell with any carbon nanotubes are separated from the carbon nanotube free cell – actually one cell at a time. The more recent information is under NDA with specific customers although we are allowed to publish when the study is completed.
Regards,
Kurt
Dear sir.
I live in Australia, I am an Australian citizen. Why is it that I cannot invest in biopact? is investing in your company only for us citizens if so, why??
Hi Laurence!
We’re on it – for now, please review the context of the challenge below.
The Regulation Crowdfunding rules were written by the SEC and designed for investors and Issuers in the United States. Unfortunately, there was no section dedicated to foreign investment. Current precedent states that if an Issuer accepts foreign investment through their REG CF offering, the Issuer must have intimate knowledge of the local securities law to ensure the investor is meeting local investing guidelines. Moreover, the Issuer must ensure the CF offering also complies with securities laws of the jurisdiction in which the securities are sold. As you can see, this can create a significant compliance burden for the Issuer and the portal who’s listed the offering.
The minimum is 300 shares correct?
Hello Menachem,
Yes, you are correct. 300 shares ($465) is the minimum.
Regards,
Kurt
Mr. Swogger:
I’ve been reading with great interest about BioPact CT and find myself cautiously optimistic about investing in this venture. Several questions came to mind when I read that BioPact CT was spun out from BioPact Ventures who licensed its rights to use MGMR to BioPact CT:
(1) Did I properly conclude after reading the materials that BioPact CT may not currently own any patents or any intellectual property? If patents or IP is developed by BioPact CT, will they remain as owned by BioPact CT or does some agreement preclude this from happening?
(2) What ongoing roles do Molecular Rebar Design and BioPact Ventures play that will impact the future income revenue streams of BioPact CT? Said another way, for each dollar of revenue earned by BioPact CT, how much will each of these parties receive before the shareholders see any benefit to the BioPact CT bottom line?
(3) What was the cost to BioPact CT for the licensing rights to use MGMR? Is it a one-time cost or is it renewable on an annual basis?
(4) Per the February 12, 2020, Offering Statement:
a. A perpetual royalty of 4% per quarter or $100,000 will be paid to BioPact Ventures, with 12% interest payable for unpaid amounts. So on top of the undisclosed licensing fee amount(s) that must be paid, am I correct in concluding that even if no revenue income is received by the 2022 operating year, BioPact CT will be obligated to pay BioPact Ventures $400,000 accruing at 12% interest starting that year?
b. Upon “mutual written agreement of the parties” the Patent Licensing Agreement can be terminated. Since 86% of the current company is owned by 4 individuals, the people who purchase common shares of BioPact CT will obviously have voting impact on the matter. What assurances can be offered to the minority stockholders that their interests will be protected from this provision that doesn’t even have a “for cause” stipulation?
c. Both listed BioPact CT Officers and Directors are shown to have previously co-founded and worked for BioPact Ventures, LLC or for Molecular Rebar Design, LLC or for both. What are their ongoing roles and ownership interests in these “previous” companies?
d. The use of proceeds section shows if fully funded, $200,000 will be used to repay a loan, apparently to BioPact Ventures and Molecular Rebar Designs. If this is the case, why does the financial statement or its accompanying notes from the CPA not disclose this amount as being a debt or liability of BioPact CT in the legal filing for Crowdfunding? Where is this debt obligation fully disclosed in your filing documents.
(5) Exactly what assets and value will BioPact CT have to sell in a future exit strategy should the opportunity arise?
Thank you for your time and I look forward to seeing your responses.
Bruce Wertz | St. Petersburg, FL
Dear Bruce,
Thank you for the interest in BioPact CT . This response took some extra time due to a question about our financials, which is answered below along with your additional questions.
BioPact CT has the exclusive rights to the patents, not the ownership. The patents cover much more than the rights to in vitro cellular transport, so direct ownership is not practical. If IP is developed by BioPact CT, it depends on what the subject matter is on who owns the patents. To make money it is not ownership of the patents, but ownership of the rights that is critical. The way the I/P is structured BioPact CT has rights for its fields from all 80+ patents in the portfolio held by the parent company. The income to BioPact CT comes to BioPact CT first, with any royalty paid to BioPact Ventures, as per your comment. Value collected by BioPact Ventures is through the royalty and ownership of a block of shares, plus a nominal amount from product sales. MRD income is from BioPact Ventures only. BioPact Ventures does sell services to BioPact CT on an ongoing basis until BioPact CT has critical mass to afford to have its own people. These services are at cost to BioPact Ventures.
The license is exclusive and perpetual as long as royalties are paid, including the minimum royalty as mentioned by your question. If the minimum is not paid there is an interest charged for late payment. The license can be terminated with mutual consent, but each party has fiduciary responsibilities to each parties’ shareholders. If BioPact CT Directors do not consider the rights of the minority shareholders, they are subject to the rules and duties imposed by governance enforced by legal and registration entities. The management of BioPact CT continues to be associated with BioPact Ventures or in one case both BioPact Ventures and MRD. They have large ownership positions in each entity as disclosed in the offering documents. When BioPact CT is large enough for a both a CEO and CTO, the current Management will convert to full time leaders. The current Management will continue to be on the Board.
The $200,000 note is disclosed in several places throughout the offering statement, including in the explanatory note on the cover page of the offering circular, in the use of proceeds section, in response to question 24 describing the indebtedness of the company and, in response to question 26 relating to related party transactions. Unfortunately, it was accidentally omitted from the financial statements. We appreciate you pointing this out to and will be filing an amendment to our offering statement with restated financial statements that address this.
As to what assets BioPact CT could sell if it chose that would be the rights to the patents and other intellectual property, any licenses signed, customer lists and relationships, rights to a supply of MGMR and any partner arrangements. If BioPact did an exit, it could either sell all shares or sell all assets depending on the needs of the acquirer and on the terms for the best deal for shareholders. The value depends on what the acquirer and BioPact CT determine the parties will agree.
Regards, Kurt
To whom it may concern:
I am a Japanese citizen and a legal US resident (I have been married to a US citizen for over 30 years). I wondered if I qualify to invest in your company even though I finished answering questions of all stages. And now my question is if I am qualified to invest in your company at this stage or not. Please advise.
Thank you.
Masataka Idomoto
Masataka,
You can absolutely invest. Please do not hesitate to call us at 800.879.7606 or email [email protected] if you need a hand.
I am awaiting the answers to Mr. Wertz’s questions,
Thank You so much for your work, my family is also affected by these disease
Hello,
This is Masataka, again.
I have two issues I would like to inquire about.
First one is that On the Agreement Step 3, there is a choice to be indicated either Accredited or Non- Accredited. I answered as a Non-Accredited investor. And yet in the last Step, there is a statement as if I am an Accredited investor. I could not mark “x” or place a check mark for a Non-Accredited investor which appear at the very last. I could not manage to do that.
I want to make sure that I am registered as a Non-Accredited investor once My check and investor identification which I already received is sent in and accepted by Prime Trust in Nevada.
Second one is just to be certain, is Prime Trust the escrow agent you are using? If it is not, then I don’t have the right information where to send the check and my investor identification information.
Please help me to be sure on these two things.
Thank you.
Hi Mastaka,
Thank you for reaching out. A member of our customer support team will help you finish up your transaction!
Have a great day.
Hi, I am interested in your company but also am waiting for your response to Mr. Wertz’s questions. They seem to be pretty relevant questions. Do you have his reply somewhere else?
Thank you for your integrity in responses.
Mr. Swogger:
Thank you for your well written responses. I appreciate the time and effort you put into providing them. However, I’m sure it was just an oversite, but you did not respond to my Question 2, which asked:
“What ongoing roles do Molecular Rebar Design and BioPact Ventures play that will impact the future income revenue streams of BioPact CT? Said another way, for each dollar of revenue earned by BioPact CT, how much will each of these parties receive before the shareholders see any benefit to the BioPact CT bottom line? ”
I’d like to know just how much of the revenue will be coming off the top to better understand how much working capital Biopact will have left for operations. What percent of gross income can shareholders expect to see paid to third-parties in total fees, expenses and royalties? Will it be a total of 8%, 10%, 12% or some other amount?
I see the design structure for Biopact following the concept of a franchise [even though it’s not] and for that reason the impact on the top line of the company is important for me to know before I can invest.
Thank you,
Bruce Wertz | St. Petersburg, FL
Hi Bruce,
Apologies for missing this question. Any income from the shares of BioPact CT and the royalties already described (BioPact CT owes a 4% royalty to BioPact Ventures beginning at the end of the first year) are payable to BioPact Ventures, and are the only major income revenue streams that go to BioPact Ventures. There is another minor income stream to BioPact Ventures in supplying MGMR. While the cost is to BioPact Ventures is 4 times what it costs MRD to make the MGMR, that cost is incidental as MRD supplies its bulk cost in kilograms (about $1600 per kg at this time). The use of MGMR per patient is milligrams per dose using MGMR. Initially MRD donated 10 kg of MGMR to BioPact Ventures, which in turn was gifted BioPact CT and will last for quite a long time.
BioPact Ventures will in turn distribute these income streams to MRD and IPRD, the primary owners of BioPact Ventures. Any services that are provided by MRD or BioPact Ventures, such as technical or administrative services, are at cost as direct expenses. As mentioned, these services will be provided by BCT itself once its size can justify the overhead.
Regards,
Kurt
Kurt:
Thank you for taking what appears to be a significant amount of time to answer some tough questions. Not only were your answers beneficial to me, but I’m sure they were to many other potential investors who are as serious about good due diligence and great transparency from the company. I look forward to becoming an investor in Biopact.
Regards,
Bruce Wertz | St. Petersburg, FL
Bruce,
Thank you for working with us to provide more clarity, not only for you, but for us as well and any other potential investors. We do all we can to be as transparent as possible both in our technology and business practices. Sometimes being too close to a situation doesn’t give you the perspective needed, so we appreciate your questions.
Thanks again,
Kurt
You are targeting the production of CAR-T and CRISPR therapeutics for your product. But the research paper specifically addressed using discrete carbon nanotubes for drug delivery into the bone/bone marrow (in the paper, specifically doxorubicin). That of course requires a lot of work with the FDA for approval. Do you ever plan on working with a company producing doxorubicin or a similar otherwise toxic chemotherapeutic for bone marrow diseases, like multiple myeloma or osteosarcoma?
Dear Fredrick,
Thank you for your interest and question.
We have worked with Doxorubicin targeted to the bone and it works well. The issues as you mention is the FDA requirements that require large amounts of investment. BioPact CT is licensed and focused on treatments such as CAR-T that do not require FDA requirements, as the MGMR never enters the body. The chemistry and cell transfer is done in vitro (outside the body) by separating the T cells from the blood. The genetic engineering, using MGMR in a cell culture, and then separating cells with MGMR from the cell mass to be injected back into the body to combat the cancer or other disease. MGMR is a specification chemical which the biotech company that is developing the treatment has to prescribe it in the process to do the CAR-T treatment, but does not have to consider the long term effects of the MGMR in the body. This avoidance of the need for considering long term effects is a way to get our technology into treatments quicker although longer term we will expand the use of MGMR for injection into the body as part of the treatment.
Regards, Kurt
If you are a Canadian, how do you invest in this company? BIOGEN
Hello,
I was curious of your take on the below I am not a scientist but wondering if it has any impact to what you and team are doing at Biopact? Is this a competitive delivery method?
Professor Emmanuelle Charpentier and Professor Jennifer Doudna have won the 2020 Nobel Prize in chemistry for their work developing a method for genome editing.
https://news.sky.com/story/two-women-jointly-win-nobel-prize-for-chemistry-for-first-time-in-history-12098161
Hi Bryan,
Thank you for the interest in BioPact CT and your question. MGMR would enable the technology created by the work that Professors Emmanuelle Charpentier and Professor Jennifer Doudna did by delivering the CRISPR into the human cell, to do the genetic engineering. In simplest terms, MGMR is a tool to make what they did easier to implement.
It was a well deserved award to both of them.
Regards, Kurt
Mr. Swogger,
I am extremely interested in investing in Biopact. I have invested in over 40 companies in the last 4 years. Please do not take this question the wrong way. I am asking it because of my investments in other stocks and knowledge in this field. I have a large position in Sangamo and regularly read all the research with Sangamo and all other Crisper type stocks. As you have stated, they are all using viruses to edit or provide gene therapy. Sangamo is also constructing a manufacturing facility at their HQ in N. CA. I know that one of the major problems has been the delivery of the Zinc fingers to the cells and they are actually better than crisper. My question is all of those companies have hundreds of scientists and molecular biologists working on a better delivery vehicle and I can’t understand how a start-up without that kind of experience or expertise could come up with something the industry as a whole, has not been able to figure out. I also keep up with what the CEO’s often present and wonder if they are aware of Biopact and if so, why haven’t they already bought Biopact out? If you could provide anymore explanation on what Biopact has done and why the other major players have not, I would greatly appreciate it. Thanks.
Dear Darren,
Thank you for the interest and the very apropos question.
In the bio-world, carbon nanotubes have been tried numerous times, mostly unsuccessfully. Therefore, most scientists did not consider it would be viable for use in cell transport. The basic reason for the lack of success of the carbon nanotubes is that they were actually micron sized bundles of many hundreds of thousands of individual, discrete nanotubes, which often led to cell damage or death. MGMR are discrete nanotubes that are a unique form and chemistry that are “eaten” (endocytosis) by cells, with no harm to the cell, and deliver whatever payload is on the nanotube into the cell. The industrial form of MGMR called Molecular Rebar, (MR) is a trademark is used in batteries, rubber, coating and so on. The applications are many. Currently one of the parent companies of BioPact CT holds over 80 patents.
The utility of MR for medicine was discovered later and was useful in the new discrete form, now referred to as MGMR. Interestingly enough, we found this attribute doing tox testing for what effect MR had on cells. There is no adverse effect we could detect, but it was noted the MR would transport into the cells. And away we went trying to find utility and MGMR and BioPact CT were born. As to letting people know what we have, the purpose for our crowdfunding is to get resources to connect to all the people who are doing cell engineering to let them know of this new tool. I hope this answers your questions.
Best regards,
Kurt
In your interview with Jordan, the question was asked about other potential industry applications. Your response mentioned batteries, rubber, coatings and 3D printers, but it was unclear to me if these are potential avenues for BioPact CT or for the parent company only.
I am a current investor and invested based on CAR-T applications. Now it seems you are also pursuing CRISPR applications as well?
Dear Dave,
Thank you for your interest and your investments.
The parent company, MRD, has developed rubber and coatings and battery additives; they are not part of the BioPactCT scope. The only thing that is common from a technology side is that they all share patents, with each market owning the right to use the patents for their particular market. The BioPactCT market includes any use outside of the body including CAR-T as well as other uses.
Practitioners of CAR-T use many types of cell engineering including CRISPR. MGMR can be used on whatever chemistry the development team develops for their particular CAR-T treatment (there are many by the way). Our focus remains on CAR-T, although if customers want to use MGMR for other uses, we will supply the samples.
As mentioned I have a personal interest in getting CAR-T more available.
Best Regards,
Kurt
Kurt- I’ve been following since day 1 of this raise to see the progression. You seem to have made some traction with partnership and amount raised to this point. Congrats.
I do have a question regarding valuation…how did you reach a 30 mil pre-money valuation for this raise?
Dear Michael,
Thank you for your interest.
A third party independent evaluator was hired before we launched our offering to determine the value of Biopact CT. We then double checked with one of our own consultants who also does valuations. They came back with just a little higher than the $30 million so we rounded it down a bit.
Best Regards,
Kurt
Kurt, I have the same question Michael P. asked. How did you arrive at your stock price valuation ?
HI William,
We appreciate your interest!
You should see our reply to Michael in the comments. Please let us know if you have any additional questions.
Best regards,
Kurt
Goodmorning Sir, As I’m reading I’m super excited to see the next wave of medical treatment. I will be investing sooner than later. One question, are your shares still available and for how long. I’m sure I have not been the only one with same question. I did read that an extention was granted to future investors due to Covid. If so how long do we have til cut off date?
Hi Lance,
The offering was extended to February 21, 2021.
Thank you for your interest!
Regards,
Kurt
Can you explain how it is that mitosis doesn’t randomly allocate some of the residual nanotubes to each of the daughter cells? I would have expected based on my understanding of the biology that there should be no reason for any of the daughter cells to end up with no nanotubes. What mechanism prevents the nanotubes from ending up one of the daughter cells?
And, if we assume that some of the daughter cells do end up without any nanotubes, how do you separate them out so that you can only return the daughter cells with no nanotubes to the patient? Are you just including an extra marker molecule in every payload?
Are either of these steps in your process covered by your patents?
Dear Jason,
Thank you for the interest and questions.
Based on our observations so far, MGMR migrates to the nucleus or the microtubules of the cytoskeleton and stays there, even during the cell splitting process. This is good for delivery of the payload, which is generally targeted to the nucleus and good for separation of the MGMR later. We can observe using electron microscopes and fluorescent tagged tubes. We can never say zero of course; but the amount transferring is non-detectable.
Separating the cells with and without MGMR will be done by a type of flow cytometry – a cell separation process and device that examines and sorts each cell using laser imaging. We have been held up in testing due to lack of access to the type of cytometer at UT San Antonio so we are looking for access to a unit here in Austin. We are assured this separation method will work as MGMR is very sensitive to electromagnetic fields; we need to determine the best conditions for separation. At this time the opinion is that we do not need a marker on the tubes, but if necessary that can easily be done. The good news is this type of separation is well known and practiced, the bad is that means it is not so patentable.
Best Regards,
Kurt
How is our investment doing?
Thank you for reaching out and your question.
As is with most new technology, especially in the biotech industry, proving advances in the technology and bringing to market take enormous amounts of time and money, hence the raising of funds. We do have some exciting advances taking place, not only with the technology but with current and potential partners. Look for a year-end update coming soon!
Kind regards,
Kurt
Hello-
In response to Bruce Wertz questions, you previously mentioned two principals MRD and BioPact Ventures having controlling interest in BioPact CT. In your prior response to Bruce’s question, I observed, you did not mention IPRD until your last response to Bruce’s question. Now you mentioned a third party IPRD: “BioPact Ventures will in turn distribute these income streams to MRD and IPRD, the primary owners of BioPact Ventures.”
Can you explain IPRD, the acronym , their association , license to Bio Pact CT, and how much impact they have on the bottom line or the patent?
Thank you, Paul
“BioPact Ventures will in turn distribute these income streams to MRD and IPRD, the primary owners of BioPact Ventures.”
Hi Paul,
Thank you for your interest in BioPact CT. I am sorry for the confusion.
MRD has the original patents and has assigned the rights to the medical field to BioPact Ventures, LLC which is owned by MRD and IPRD, LLC.
IPRD is majority owned by Randy Kinsel and has no patent rights. IPRD is the name of the entity. BioPact Ventures, LLC owns 19,000,000 shares of BioPact CT along with anyone who invested in the crowd funding round. Any distributions from BioPact CT are shared by the shareholders of BioPact CT, so any net income from BioPact CT is distributed to BioPact Ventures based on the share ownership which in turn distributes it to MRD and IPRD based on their ownership positions.
I hope this helps.
Regards,
Kurt
Good evening. Do you have any idea as to when this company might go public or offer its ipo?
Thank you
Hello Georges,
Thank you for your question. Our plans for the future are to grow as much as reasonably possible so that we have options in 2-4 years to take the company to the next level. When we get there, we will carefully evaluate all opportunities and do what is in the investors’ best interest. This may be a public offering, or it may be an acquisition by a larger company, or we can continue to grow the company and pay dividends to shareholders as we grow.
Regards,
Kurt
When is it expected that the IPO will happen?
Hello Tallman,
Please see the reply above, as it relates to your question as well.
Thank you,
Kurt
Hello!
I am very interest in investing but in order to invest, my custodian needs some additional documents from the company including good standing documents and bylaws. How would I go about getting additional documents like this?
Good Morning Mr. Mohr!
We’ll have a customer success agent reach out to you right away.
Have a great Day.
Hi, just checking as to when an end of year update may come out? Thank you
It says above: “The last date of the offering at this time is June 30, 2020.”
However, the “Invest Now: blue button is still live!
Is this closed or open?
Also, the valuation is current, right?
What is the projected market and valuation when fully realized? Thanks!
Hello Barry,
Thank you for your interest and we appreciate your questions.
Our offering is still open, and you can still invest. It closes on Friday, February 12th.
Our current valuation (established by an independent third party) is $30M. As I have mentioned in previous emails and in the video, I have a very personal connection and conviction in getting this to market. However, in an attempt to keep our attorneys happy, I cannot speak to the future market valuation..
Regards,
Kurt Swogger
what is the trading symbol?
Hello Herman,
BioPact CT is currently a privately held company, thus there is not a stock symbol at this time.
Kind Regards,
Kurt Swogger
Hi I am interested. I see the share price is $1.55 a share. Is there any type of discount on share price for investing higher dollar amounts?
Thanks John
Hi John,
Thank you for question and your interest in BioPact CT.
We are not able to give a discount with this offering, but we will welcome your investment should you decide to do so.
Regards,
Kurt
how do I invest?
Hi Eric,
Thanks for your interest! Simply click on the “Invest Now” button and follow the prompts.
Also, please note, our current offering ends this Friday, February 12th.
Regards,
Kurt
Am I correct as to your most recent amendment…
Did the entity your company owes $200K to opt for the equivalent in shares instead of a cash repayment?
If so, can you tell me the thought process behind that? I have an idea but would love confirmation.
Dear Scott,
Thank you for the message and interest. BioPact Ventures did opt to buy shares in BioPact CT rather than take cash for the $200,000 note. BioPact Ventures decided it would rather have the shares, and to leave more money in BioPact CT. BioPact Ventures thinks BioPact CT is a great investment, and truth be told, there was not much more thought than that at the time.
We are keeping all investors appraised of progress or changes to allow for further investment as BioPact Ventures elected to do.
Regards,
Kurt Swogger
I opened an Equifund account but don’t see BioPact CT listed. I see where your deadline has been extended but you still aren’t listed. It is now2-19-21 and the cutoff date is now 2-21-21 and I don’t want to miss it. If I do miss out since it is the weekend could us Texans that were without power, phone and water for most of the week please have an extra day to invest?
[url=https://chimmed.ru/manufactors/catalog?name=Biotech+AB]Biotech AB[/url]
Tegs: Biotrend https://chimmed.ru/manufactors/catalog?name=Biotrend
[u]tmh-medizinhandel.de[/u]
[i]toku-e eu n.v.[/i]
[b]toronto research chemicals, inc[/b]